Complete IPQC Tests for Pharmaceutical Tablet Dosage Form
What you’ll learn
-
Inprocess Quality Control Test For tablet Dosage form
-
Pharmaceutical Testing
-
Neccessity of IPQC tests
-
How to maintain quality of pharmaceutical product
Description
The development of a drug product is a lengthy process involving drug discovery, laboratory testing, animal studies, clinical trials and regulatory registration.
To further enhance the effectiveness and safety of the drug product after approval, many regulatory agencies such as the United States Food and Drug Administration (FDA) also require that the drug product be tested for its identity, strength, quality, purity and stability before it can be released for use. For this reason, pharmaceutical validation and process controls are important in spite of the problems that may been countered.
Process controls include raw materials inspection, in-process controls and target so for final product. IPQC stands for in process quality control. These are checks that are carried out before the manufacturing process is completed. The function of of in-process controls is monitoring and if necessary adaption of the manufacturing process in order to comply with the specifications.
This may include control of equipment and environment too. In-process materials should be tested for identity, strength, quality and purity as appropriate and approved or rejected by the quality control unit during the production process.
Rejected in-process materials should be identified and controlled under a quarantine system designed to prevent their use in manufacturing
DEFINITION OF IPQC
IPQC stands for IN PROCESS QUALITY CONTROL .
These are checks that are carried out before the manufacturing process is completed. The function of of in-process controls is monitoring and if necessary adaption of the manufacturing process in order to comply with the specifications .this may include control of equipment and environment too. In-process materials should be tested for identity, strength, quality and purity as appropriate and approved or rejected by the quality control unit during the production process. Rejected in-process materials should be identified and controlled under a quarantine system designed to prevent their use in manufacturing .Written procedure should be established and followed that describe the in-process controls and tests as specified
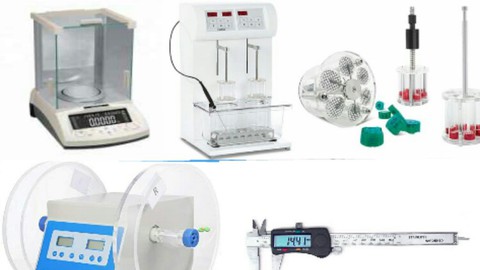
QUALITY CONTROL EQUIPMENTS
- Friabilator
- Disintegration apparatus
- Hardness Tester
- Vernier Caliper
Course Eligibility :
Diploma Pharmacy Students
Bachelor of Pharmacy Students
Master of pharmacy Students
Pharmaceutical industry
Pharma Professionals
Benefits of course:
- Learning of IPQC Tests
- Tablet Dosage quality control
- Hadling of Equipments
- Theory & Practical demonstration
- Certificate of completion
Who this course is for:
- Pharmacy
- Pharma Professionals



